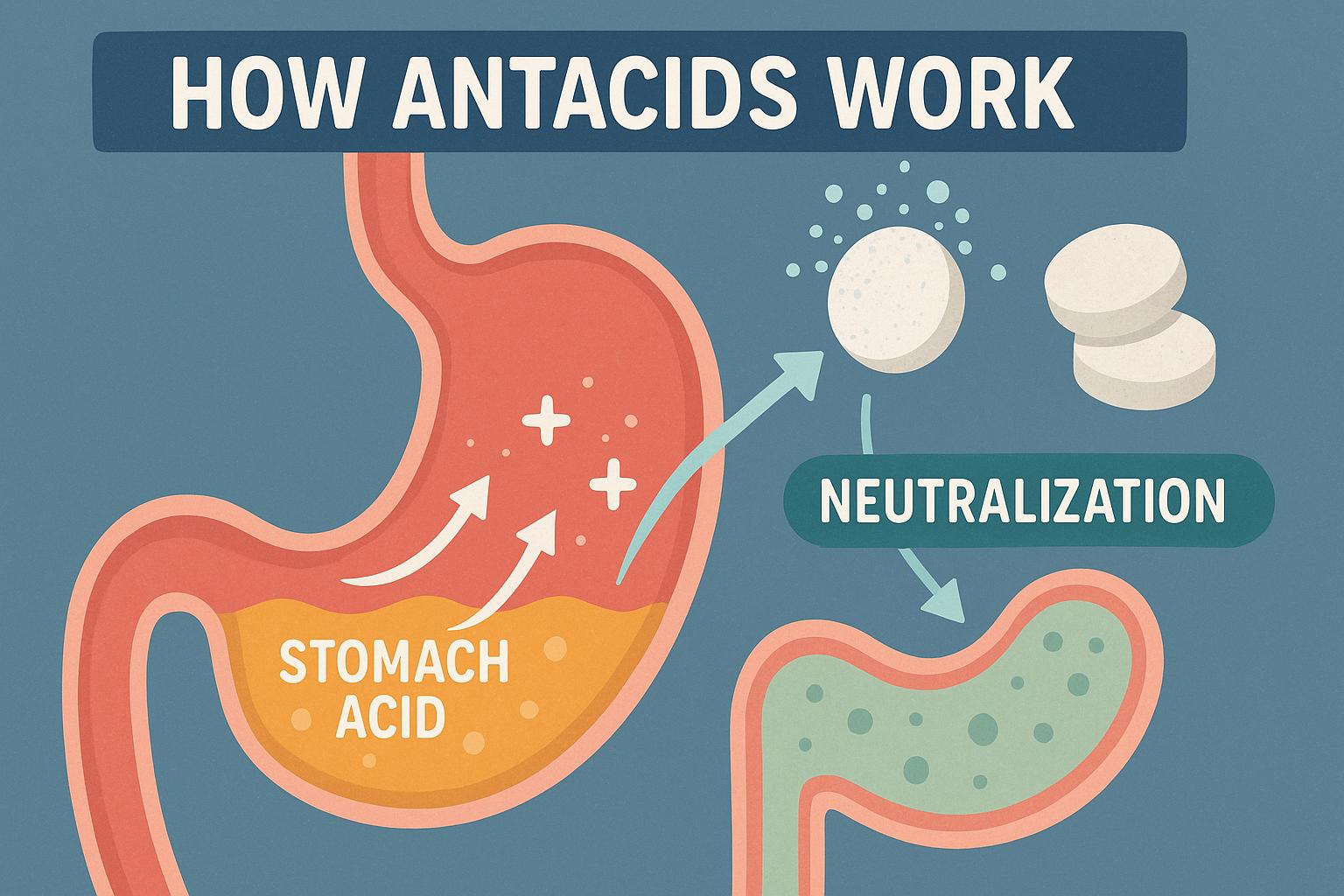
How antacids work to neutralize stomach acid.
Understanding Antacids and Their Role in Neutralizing Stomach Acid
The human stomach naturally produces hydrochloric acid, a crucial component in the digestive process that helps break down food and eliminate potentially harmful microbes that enter the digestive system. Despite its essential role, when produced in excess, this acid can lead to uncomfortable health conditions such as heartburn, acid indigestion, or an upset stomach. Antacids are a common and effective remedy employed to address these discomforts by neutralizing stomach acid and providing relief from associated symptoms.
Composition of Antacids
Antacids are composed of basic compounds designed to react with and neutralize excess stomach acid. The key active ingredients typically found in antacids include:
Calcium carbonate: This compound acts as a potent antacid, effectively neutralizing stomach acid while also serving as a dietary calcium supplement.
Magnesium hydroxide: Often referred to as “milk of magnesia,” magnesium hydroxide serves as both an antacid and a laxative. Its effectiveness in neutralizing acid is well-documented, though it can also impact bowel movements.
Aluminum hydroxide: This compound is often used in combination with magnesium hydroxide to counteract its laxative properties, striking a balance that can help control symptoms without causing diarrhea.
Sodium bicarbonate: Commonly known as baking soda, sodium bicarbonate neutralizes stomach acid rapidly, offering quick relief. However, it can cause a rebound effect, where acid production increases after the initial neutralization.
These ingredients work synergistically to temporarily neutralize gastric acid, increasing the stomach’s pH level and reducing the irritation caused by the acid’s corrosive nature.
How Acid Neutralization Works
Acid neutralization is a fundamental chemical reaction where a base (or alkaline substance, such as those contained in antacids) interacts with an acid to form water and a salt, thereby decreasing the acidity in the stomach. The foundation of this process can be observed through chemical equations that illustrate how specific compounds interact. For instance, the neutralization reaction between hydrochloric acid (HCl) and magnesium hydroxide (Mg(OH)₂) can be expressed as follows:
Mg(OH)₂ + 2HCl → MgCl₂ + 2H₂O
This equation clearly demonstrates the neutralization process. Through this interaction, magnesium chloride (MgCl₂), a salt, and water are produced, helping to alleviate the acidic conditions within the stomach environment. By reducing the overall acidity, these reactions can significantly ease symptoms associated with excess stomach acid.
Forms of Antacids
Antacids are produced in a variety of forms to cater to different preferences and needs. These include chewable tablets, liquid suspensions, and effervescent tablets. Each form is engineered to deliver the active compounds effectively, allowing for flexibility in how individuals choose to consume them. The choice of form does not significantly affect the efficacy of antacids, offering consumers the liberty to select products based on their preferences or convenience.
Chewable tablets: These are favored for their convenience and ease of use, making on-the-go relief a viable option.
Liquid suspensions: Often provide rapid relief due to their ability to coat the stomach lining quickly.
Effervescent tablets: These are dissolved in water before intake, making them a good choice for those who prefer liquid medicine.
Side Effects and Considerations
Though antacids are generally an effective means for alleviating symptoms of excessive stomach acid, they can lead to certain side effects. It’s important to monitor usage and consult healthcare providers when symptoms persist over long periods. Common side effects associated with specific antacids can include:
Diarrhea: Excessive usage of magnesium-based antacids may lead to diarrhea due to magnesium’s natural laxative effect.
Constipation: Aluminum-containing antacids may cause constipation because of their astringent effects on the gastrointestinal tract.
Besides these side effects, it’s crucial to consider potential interactions with other medications. Antacids can alter the absorption and efficacy of specific medications, necessitating careful planning of their consumption. For example, antacids can interfere with the absorption of certain antibiotics and other medications requiring an acidic environment for optimal absorption.
Users should strictly adhere to dosage recommendations as indicated by healthcare providers or the product packaging. Overuse can lead to an imbalance in electrolytes, particularly if sodium bicarbonate or other sodium-based compounds are used in excess. Users with specific health concerns, such as kidney disease or hypertension, should exercise caution and consult a healthcare provider before using antacids due to the potential for adverse interactions or exacerbation of existing conditions.
Exploration of antacids involves understanding both their mechanism of action and their potential effects, rendering them a valuable tool for managing digestive discomfort. For those seeking more in-depth knowledge about antacids, their uses, and safe practices, resources from reputable health organizations or academic institutions can provide deeper insights and guidance, contributing to informed and safe usage of these medical aids.
